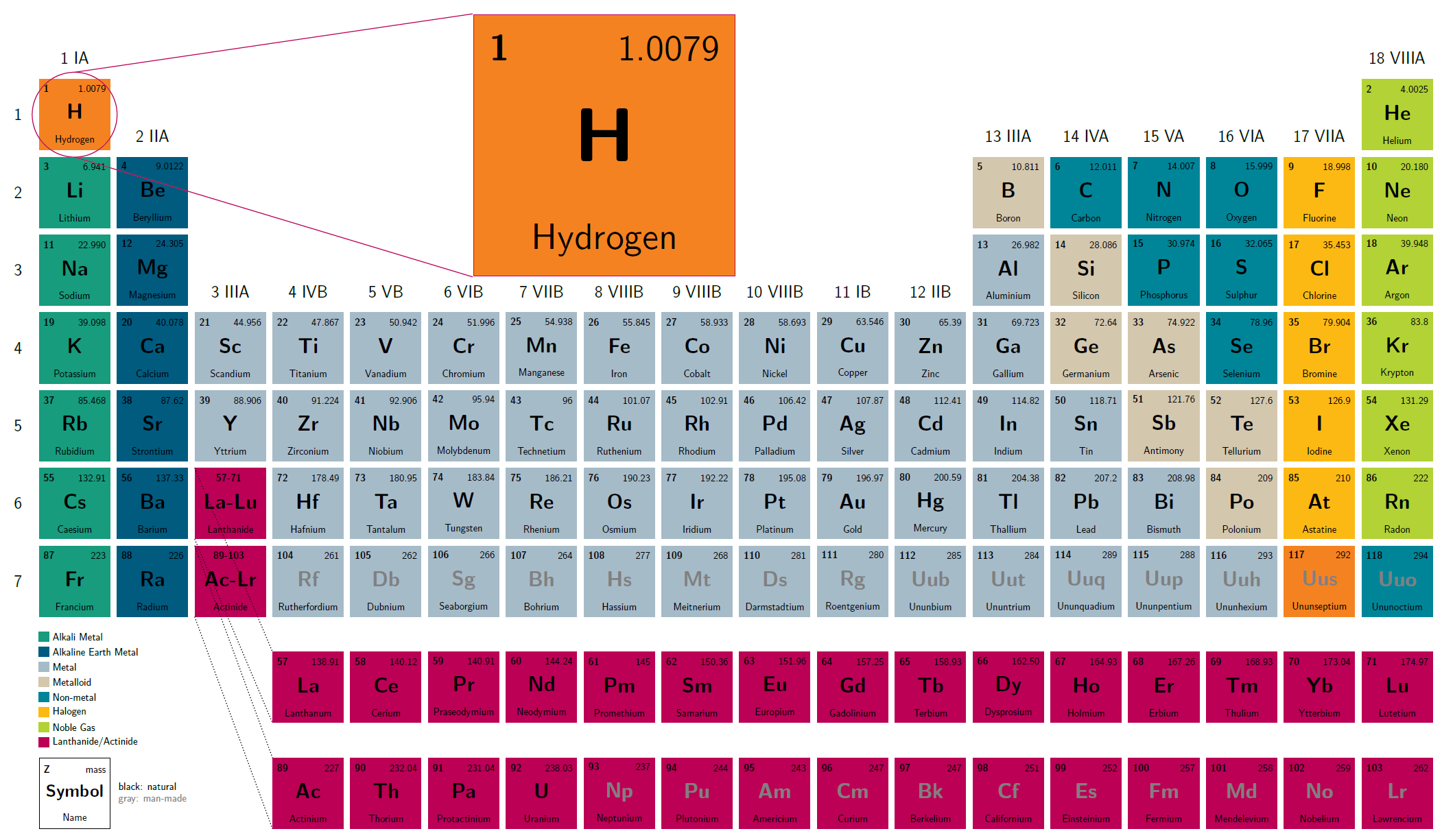
The element hydrogen occupies the first place, top left, in the periodic table of the elements. This is because it consists of one proton and one electron and thus has the lowest atomic mass of all elements. As the most common element in the universe, hydrogen is involved in all organic compounds, among other things. On Earth, it occurs virtually exclusively in chemical compounds or as molecular hydrogen, H2. Probably the best known chemical compound of hydrogen is water (H2O). So-called hydrocarbons such as methane (CH4), the main component of natural gas, and petroleum are other prominent representatives of the compounds of hydrogen. Carbohydrates such as glucose (C6H12O6) also contain bound hydrogen. Thus, hydrogen is also a component of the carbon cycle and photosynthesis: all biomass contains hydrogen. In addition, more than half of all mineral compounds do as well contain hydrogen.