Hydrogen has an energy density of 33.3 kWh/kg and is produced with an efficiency of approx. 50 - 80 %, depending on the type of electrolyser and its mode of operation. Accordingly, approx. 42 - 66.7 kWh of energy must be used to produce one kg of H2. The following section describes the production of hydrogen by water electrolysis and the most common types of water electrolysis.
In water electrolysis, water (H2O) is split into hydrogen (H2) and oxygen (O2) by applying an electrical voltage. Thus, it is an electrochemical reaction. The reaction equation of water electrolysis is: [1, 2]
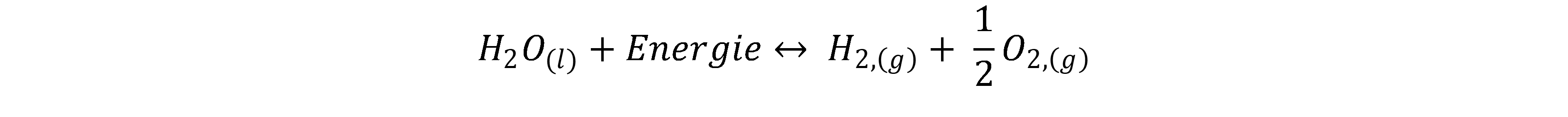
The reaction is divided into two half-cell reactions, which take place at the cathode and anode respectively. The two electrodes are immersed in an electrolyte. The most common types of water electrolysis are characterised by the type of electrolyte used.
These types of electrolysis are:
- Polymer-Electrolyte-Membrane-Electrolysis (PEM)
- Alkaline Electrolysis (AEL)
- Solid-Oxide-Electrolysis (SOEC, Solid-Oxide-Electrolysis-Cell)
Polymer-electrolyte-membrane-electrolysis (PEM)
In PEM electrolysis, a proton-conducting, gas-impermeable membrane is used. Liquid water is fed to the anode where it is converted into oxygen and protons (H+). [2, 3]
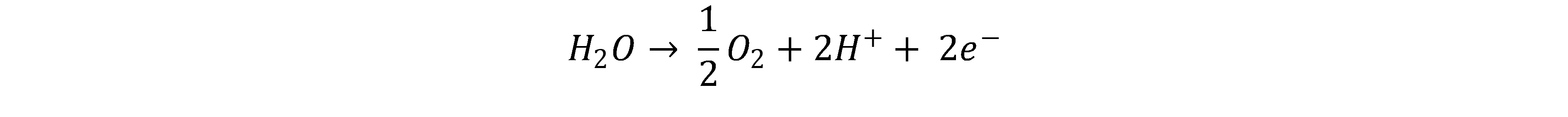
The protons diffuse through the membrane to the cathode where they are combined with electrons to form hydrogen[2, 4]
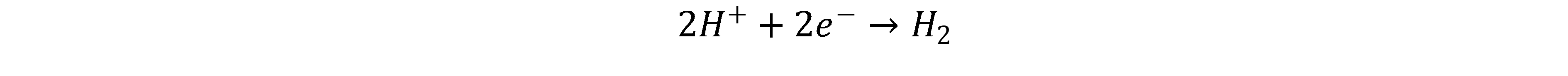
The advantage of PEM electrolysis is its highly dynamic behaviour, which is more suitable for coupling with fluctuating renewable energy sources compared to AEL. In addition, a high purity of the hydrogen is given even in partial and overload operation. In addition, higher current densities are possible compared to the AEL. The disadvantages include the high costs and the need for expensive and rare catalyst materials. Platinum, iridium and ruthenium should be mentioned here. These are needed because they are the only materials that are active and stable over long term in the acidic environment of the PEM. [3]
Alkaline-Electrolysis (AEL)
In alkaline electrolysis, the water is usually split at two nickel-based electrodes. These are separated by a diaphragm, which prevents the passage of gas bubbles but allows the electrolyte, potassium hydroxide solution (KOH(aq)), to pass through. [2, 4]
In alkaline electrolysis, hydroxide ions and hydrogen are formed from water at the cathode by accepting electrons. [3, 4]

Due to the charge difference, the hydroxide ions move to the anode and react there, releasing electrons to form water and oxygen. [2, 4]
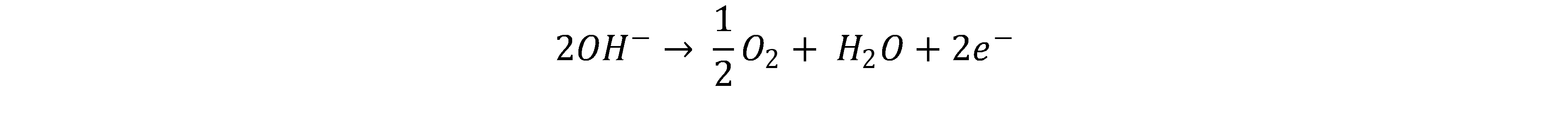
Since alkaline electrolysis has a high market readiness, the investment costs are low compared to PEM. In addition, alkaline electrolysers have very good long-term stability. The alkaline environment allows the use of cheaper materials; there is no need for rare noble metals.
The disadvantages of the AEL are the low current density compared to PEM electrolysis, which leads to larger overall plants. In addition, there are problems in the lower load range with dynamic operation of the AEL.
Solid-Oxide-Electrolysis (SOEC)
Solid oxide electrolysis cell (SOEC) typically uses an oxide ion conducting (O2-) ceramic as the electrolyte and separator. This only becomes sufficiently conductive above several hundred °C, which is why SOEC is usually operated between 500 - 900 °C and thus represents a form of high-temperature electrolysis. In SOEC, water vapour is split into hydrogen and oxide ions at the cathode. [5]

The oxide ions diffuse through the solid electrolyte and are oxidised to elemental oxygen at the anode.
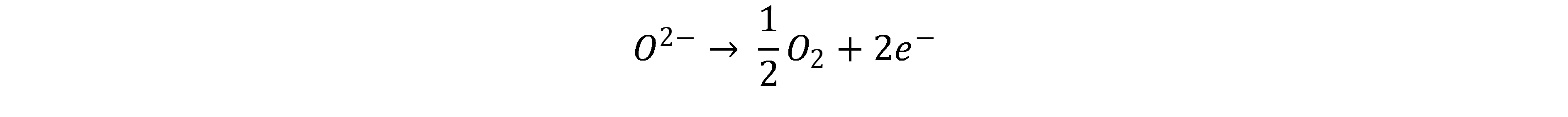
One advantage of SOEC is its higher efficiency compared to PEM and AEL. This results from the temperature dependence of the free enthalpy of reaction; at higher temperatures, electrical energy can increasingly be substituted by thermal energy. Depending on the operating point, this thermal energy can be produced itself or supplied from outside. If heat is supplied from outside, efficiencies >1 can be achieved in relation to the amount of electrical energy used.
However, the high temperatures also lead to increased material stress (especially during dynamic operation) and long start-up and shutdown times. Compared to alkaline electrolysis, SOEC is still relatively new and has to catch up, especially in terms of long-term stability. In terms of resource availability, SOEC is ranked between PEM and AEL; noble earths are needed, which are not as expensive as precious metals, but could lead to strategic dependence.
References:
- Shiva Kumar, S.; Himabindu, V. Hydrogen production by PEM water electrolysis – A review. Materials Science for Energy Technologies 2019, 2 (3), 442–454. DOI: 10.1016/j.mset.2019.03.002.
- David, M.; Ocampo-Martínez, C.; Sánchez-Peña, R. Advances in alkaline water electrolyzers: A review. Journal of Energy Storage 2019, 23, 392–403. DOI: 10.1016/j.est.2019.03.001.
- Universität Augsburg. Polymerelektrolytmembran - Elektrolyse (PEM). https://www.uni-augsburg.de/de/forschung/einrichtungen/institute/amu/wasserstoff-forschung-h2-unia/h2lab/h2-er/elektrolyse/pem/ (accessed 2023-04-03).
- Universität Augsburg. Alkalische Elektrolyse (AEL). https://www.uni-augsburg.de/de/forschung/einrichtungen/institute/amu/wasserstoff-forschung-h2-unia/h2lab/h2-er/elektrolyse/ael/ (accessed 2023-04-03).
- Universität Augsburg. Hochtemperatur (Feststoffoxid) Elektrolyse. https://www.uni-augsburg.de/de/forschung/einrichtungen/institute/amu/wasserstoff-forschung-h2-unia/h2lab/h2-er/elektrolyse/th-el/ (accessed 2023-04-03).